Guide number 4217/04.02.00.01/2021
1 Preface
Materials and articles intended to come into contact with food, later referred to as food contact materials (FCM), are materials and articles designed to come into direct or indirect contact with foods. Such materials include, for example, food packaging materials, kitchenware and tableware, small kitchen appliances, cardboard tableware, disposable gloves, industrial food manufacturing equipment, such as pipes, hoses, seals, pumps, as well as also food containers and food tanker trucks. Products used in the manufacture of FCM, such as semi-manufactures, lacquers, adhesives and printing inks are also food contact materials. FCM can be e.g. plastic, paper, cardboard, metal, and mixtures of these, ceramics, cellophane, rubber, silicone, ion exchange resins, wood, stone, leather, or fabric.
Responsibility for the safety of FCM rests with all the operators in supply and application chain (operators who manufacture or import these materials or have them manufactured, wholesale distributors/marketers and food establishments). The authority controls the achievement of compliance for the products through controls of the operators' processes and in-house control, as well documentation demonstrating compliance, and occasionally through sampling.
The safety of packaging materials and other FCM is part of food safety. The safety of FCM is of wide significance, as all foods come into contact with different materials and articles at several phases of the production chain.
The Finnish Food Act (297/2021) also applies to materials and articles coming into contact with food. The compliance of FCM on a general level is provided for in EC Regulation No 1935/2004[1] on materials and articles intended to come into contact with food (later: Framework Regulation). In addition, some material-specific provisions, also referred to as specific measures, have been adopted. The ultimate purpose of legislation is to minimise the quantity of chemicals transferred from the materials and articles to foods to a level where they are not harmful to human health.
These guidelines are designed for the authorities referred to in the Finnish Food Act to ensure the safety of FCM, the use of consistent food safety control procedures, and the promotion of equal treatment for operators. These guidelines will also be useful to the operators of the sector when they develop the quality management system or the in-house control plan required by good manufacturing practice.
The actions of authorities must be based on the authority conferred on them by law and laws must be strictly complied with in the actions of authorities. Regulatory guidelines are not, by their legal nature, binding on other authorities or operators. Issues pertaining to the application of legislative regulations are in the last instance settled by a court of law. This Guide presents both direct quotations from legislation and interpretations on the application of legislation. Quotations from legislation are in italics. The interpretations presented in this Guide constitute Finnish Food Authority's views on how legislative regulations should be applied.
2 General requirements spesified for food contact materials
2.1 Scope of application of provisions on food contact materials
The scope of application of provisions on FCM is defined in Article 1 of the Framework Regulation:
The Regulation shall apply to materials and articles intended to come into contact directly or indirectly with food and which in their finished state:
- are intended to be brought into contact with food
- are already in contact with food
- can reasonably be expected to come into contact with food.
The provisions on FCM shall not apply to:
- antiques
- covering or coating materials which form part of the food and may be consumed together with this food (e.g. cheese rinds, ice-cream cones)
- fixed public or private water supply equipment.
2.2 General safety requirements for all food contact materials
The compliance and safety of FCM comprise the following factors:
- Chemical safety in terms of composition (ingredients and additives, aids, impurities or reaction intermediates formed in the production process, or decomposition or reaction products) and the documentation demonstrating this
- Test data or calculated data, and information on organoleptic quality
- Implementation of traceability
- Data on the material to be provided to the next industrial customer (Declaration of Compliance) or labelling on sales packages of products intended for consumers.
Article 3 of the Framework Regulation lays down general safety requirements for all FCMs and articles, regardless of the material they are manufactured from:
they shall be manufactured in compliance with good manufacturing practice so that, under normal or foreseeable conditions of use, they do not transfer their constituents to food in quantities which could:
- endanger the health of the person consuming the food, or
- bring about an unacceptable change in the composition of the food, or
- bring about a deterioration in the organoleptic characteristics thereof.
In addition: The labelling, advertising and presentation of a material or article shall not mislead the consumers.
Annex III to Regulation (EC) No 853/2004 on specific rules for food of animal origin further requires that packaging materials must not be a source of contamination for the product packed in them.
2.3 Information to be provided on food contact materials, and labelling
Pursuant to Article 15 of the Framework Regulation, adequate information for use shall be provided on FCM.
Products intended for marketing to consumers (sold for money or delivered free of charge) which are not yet in contact with food shall be accompanied by:
- the words “for food contact” or a specific indication as to their use, or the symbol reproduced in Annex II; This information shall not, however, be obligatory for any articles which, because of their characteristics, are clearly intended to come into contact with food (e.g. a coffee cup), and
- if necessary, special instructions to be observed for safe and appropriate use, and
- the name or trade name and, in either case, the address or registered office of the manufacturer, processor, or seller responsible for placing on the market established within the Community, and
- adequate labelling or identification to ensure traceability of the material or article, as described in Article 17.
- in the case of active materials and articles, information on the permitted use or uses and other relevant information such as the name and quantity of the substances released by the active component so as to enable food business operators who use these materials and articles to comply with any other relevant Community provisions or, in their absence, national provisions applicable to food, including the provisions on food labelling.
As a rule, at the retail stage this information shall be displayed on the materials and articles or on their packaging, or on labels affixed to them.
In addition, the special labelling requirements of Regulation (EC) Regulation (EC) No 450/2009 apply to active and intelligent food contact materials.
In the supply chain between the FCM sector and food sector operators, the information is submitted with Declarations of Compliance (Article 16) or comparable documents demonstrating compliance.
2.4 Declaration of Compliance
The safety and compliance of materials and articles intended to come into contact with food, as referred to in the Framework Regulation, is demonstrated with documentation. Pursuant to Article 16 of the Framework Regulation, "The specific measures referred to in Article 5 shall require that materials and articles covered by those measures be accompanied by a written declaration stating that they comply with the rules applicable to them".
It has been elaborated in specific measures (e.g. (EU) 10/2011, later the Plastics Regulation) that this declaration shall accompany the products at all marketing stages other than the retail stage. This requirement is applied also to other materials except ceramics, where the declaration must be available also at the retail stage.
The form for the Declaration of Compliance is confirmed for plastic materials and articles in Article 16 of and Annex IV to the Plastics Regulation, for recycled plastic materials in EU Regulation 282/2008 on recycled plastic materials and articles, and for ceramic products (Decree 165/2006 of the Finnish Ministry of Trade and Industry).
2.4.1 Declaration of Compliance for plastic materials
The EU Commission has issued guidance on the content of the Declaration of Compliance for plastic food contact materials at various stages of the supply chain. It is available in Finnish as well as in all other EU languages.
2.4.2 Declaration of Compliance for ceramics
Ceramic articles which are not yet in contact with food shall at all marketing stages,
including the retail stage, be accompanied by a written Declaration of Compliance. The Declaration shall be made available at the point of sale to the control authority on request. The authority can give a reasonable time of 1-2 weeks for the submission of the document.
The Declaration shall have been issued by the manufacturer or a marketer operating within the EU, and shall provide the following information specified in Annex 3 to the Finnish Ceramics Decree:
- the name and address of the company manufacturing the final ceramic article and of the company that imports it to the Community
- the identification of the ceramic article
- the date of issue of the Declaration
- confirmation of the compliance of the ceramic article with the requirements laid down in Decree 165/2006 of the Finnish Ministry of Trade and Industry and Regulation (EC) No 1935/2004. The statement of confirmation can read, for example: "This ceramic article meets the requirements of Decree 165/2006 of the Finnish Ministry of Trade and Industry and Regulation (EC) No 1935/2004.”
2.4.3 General guidelines for the content of the Declaration of Compliance
Although the number of specific measures adopted at EU level with respect to FCM is still limited, some Member States have issued national provisions or guidelines regarding the issuance and content of a Declaration of Compliance document for all materials and articles intended to come into contact with food. Also in Finland, the Finnish Food Authority has outlined a policy stating that a document demonstrating compliance must accompany the material or article. In the case of regular, contract-based supply of FCM, the submission of documents demonstrating compliance at the start of the deliveries and then once every three years after that is adequate, as well as whenever any changes take place in the composition of the FCM, or the food sector operator has defined the frequency of submission in its in-house control plan. In individual orders/deliveries, the document demonstrating compliance should accompany each order/delivery.
The Declaration of Compliance must include the following information:
- name and address of the operator responsible for the document
- date of issue of the document
- identification code/construction of materials/articles
- confirmation on compliance of material/article with the safety requirements of Regulation (EC) No 1935/2004, and chosen safety references and also that it has been produced following the good manufacturing practice referred to in Regulation (EC) No 2023/2006[2] (including required tests)
- when the material contains substances that are subject to restrictions: adequate information to allow subsequent operators in the chain to take them into consideration when to manufacture products compliant with requirements
- when the material contains dual use additives: adequate information (name, quantity) to allow the food manufacturer to take them into consideration in their activities
- suitability of material/article for food contact use:
- type of food the material is suitable for contact with
- time and temperature conditions for which the material is suitable during manufacture and storage
- ratio of contact surface area to volume of the material, on which the tests for determination of compliance were based
- for paper and cardboard: information on whether virgin or recycled fibre has been used, and information on bleaching (if applicable),
- for plastic materials: the requirements for additional information referred to in Regulation (EC) 282/2008 on recycled plastic materials, such as the EU Register number of the recycling process
- information on any use of a barrier layer.
The operator can make the documentation accessible and available to the customers in electronic form, provided traceability is adequately addressed.
The information provided in the Declaration of Compliance should be limited to the fulfilment of the requirements laid down in FCM legislation. References to waste laws or environmental legislation, for example, may mislead a food sector operator. The maximum limits specified for chemical ingredients in waste laws and environmental legislation are often significantly higher and do not indicate if the quantity ensures the safety of the material in food contact. If the fulfilment of requirements made in this legislation is to be indicated in the same document as the fulfilment of the requirements of FCM legislation, a clear distinction shall be made between them with headings that prevent any risk of misunderstanding.
2.4.4 Tests for demonstration of compliance
As a rule, tests and calculations of the migration and/or total concentration of substances as well as the migration of odour, taste and any colour to the food or to a test substance imitating food are required to demonstrate the compliance of a food contact material. Conducting the tests is the obligation and responsibility of the operator in the FCM sector. The type of tests that are required depends on the material, its construction and intended application. Specific measures, such as the regulations on plastic and ceramic materials, indicate the requirements the fulfilment of which shall be demonstrated by tests (the overall migration limit, and if applicable, the specific migration limit of a constituent). Evidence based on tests is also needed to demonstrate the safety of materials on which there is no legislation (e.g. paper and cardboard). This is addressed in publication 2008/515 of the Nordic Council of Ministers, for example.
According to the Plastics Regulation, testing of specific migration limits is not mandatory if model calculations can demonstrate that even the complete migration of the residue of the substance in the article cannot exceed the specific migration limit. To demonstrate that a product does not meet requirements, the assessed migration value shall always be verified through practical tests.
In the testing of plastic and ceramic articles in particular, the worst case scenario can be applied. This means a product with the highest ratio of contact surface area to volume is selected for testing from among articles manufactured from similar materials, identical in shape and intended for the same application. In practice this is the smallest product in a range of products of identical shape.
The Customs Laboratory is the national reference laboratory in Finland in the field of FCM testing. The reference laboratory for the EU Commission is Joint Research Centre (JRC) in Italy.
There are several laboratories on Finland that carry out FCM testing. Also elsewhere in Europe, there is also a host of testing institutes specialising in the FCM sector can be used in testing. Only laboratories designated as official control laboratories or the national reference laboratory should be used for the examination of official control samples.
2.5 Traceability
According to Article 2 of the Framework Regulation, "traceability" means the ability to trace and follow a material or article through all stages of manufacture, processing and distribution;
According to Article 17 of the Framework Regulation:
The traceability of materials and articles shall be ensured at all stages in order to facilitate control, the recall of defective products, consumer information and the attribution of responsibility.
Operators in the sector shall have in place systems to allow identification of the businesses from and to which materials or articles and substances or products used in their manufacture have been supplied (one step forward and one step back). This information shall, on request, be made available to the competent authority.
For the retention period of documentation related to the traceability of packaging materials, the same principle based on the ”shelf life” of food may be applied as for the retention period of documentation defined in the food traceability guidelines.
3 Product-spesific requirements
3.1 Product-specific EU regulations and Finnish national regulations
In addition to the Framework Regulation applying to all materials, some regulations applying to specific products or substances have also been issued, for example:
- Commission Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food,
- Decree 165/2006 of the Ministry of Trade and Industry on ceramic materials and articles intended to come into contact with food,
- Decree 697/2005 of the Ministry of Trade and Industry on regenerated cellulose (cellophane) intended to come into contact with food,
- Commission Regulation (EU) No 1616/2022 on recycled plastic materials and articles intended to come into contact with food,
- Commission Regulation (EC) No 450/2009 on active and intelligent materials and articles intended to come into contact with food,
- Commission Regulation (EC) No 1895/2005 on the restriction of use of certain epoxy derivatives,
- Decision 268/1992 of the Ministry of Trade and Industry on the transfer of certain heavy metals from materials and articles intended to come into contact with food,
- Decision 903/1994 of Ministry of Trade and Industry on the transfer of N-nitrosamines and/or N-nitrosatable substances from materials,
- Commission Regulation (EU) 2018/213 on the use of bisphenol A in varnishes and coatings.
The most recently updated version of all regulations is applied. Finnish legislation has been harmonised with EU legislation, with the exception of the aforementioned decision 268/1992 of the Ministry of Trade and Industry which is a national regulation. This so-called heavy metal decision 268/1992 (KTM) applies to all FCMs. It provides limits for the transfer of lead, nickel, chrome and cadmium from food contact materials to food. However, testing in accordance with the decision is not required for materials or articles for which there is material-specific EU-level specific legislation or which are already legally on the market in an EU Member State. An operator may submit a self-declaration to the authority as assurance that the product is legally on the market in another Member State.
The complete legislation on FCM to be complied with in Finland has been compiled on the Ministry of Agriculture and Forestry website and on the Finnish Food Authority website. The surest way to find the most recent consolidated* version of EU legislation is on the Eur-Lex website. Enter the number and year of the act in the search field and choose the type of act (regulation, directive, etc.).
The EU Commission has also prepared guidance on the application of the Plastics Regulation. It is available in Finnish and also in other EU languages.
3.2 Materials and articles with no common EU legislation
Even though material specific, also in the case of these materials and articles, operators are responsible for the safety of the materials and articles they place on the market, based on Article 3 of the Framework Regulation. Several safety reference and self-monitoring sources for non-harmonized materials are listed below. Links to them can be found on the Food Authority website “Useful online links about contact Materials”.
The European Commission’s testing laboratory, the JRC testing laboratory has prepared a report on the legislative and commercial status of non-harmonised food contact materials. The Council of Europe EDQM has developed a general resolution for food contact materials, supplemented by material-specific technical guidance.
Paper and board form the most significant group of FCM without an EU-level specific measure or a national regulation in Finland. Other materials without common EU provisions include printing inks, adhesives, lacquers, metals, rubber, silicone, stone, wood, leather and fabric.
In Finland, publication 2008:515 of the Nordic Council of Ministers titled "Paper and Board in Food Contact" can be used as guidance related to paper and board. Likewise, the recommendations issued by the German Federal Institute for Risk Assessment (Bundesanstalt fur Risikobewertung BfR) can also be utilised (they include baking paper, filter paper, absorbent pads. BfR also has recommendations on the requirements for silicone and waxes as well as for many other non-harmonised food contact materials. The Council of Europe (EDQM) has developed a technical guide for paper and board, which can also be used as a safety reference.
Switzerland has adopted national provisions for printing inks used on food packaging, and the European Printing Ink Association (EUPIA) has been working on GMP guidance for printers (own-check guidance).
The Nordic Council of Ministers has published guidelines related to printing inks, TemaNord 2012:521, as well as check lists for control authorities, the printing ink industry and trade, and the food industry. The safety of FCM is a complex issue as far as printing inks are concerned, partly because of the critical manufacturing phases included in the printing process. The report briefly presents printing processes as well as some of the control points of the processes. This information is considered useful in control and in the operators' quality assurance activities. The recommendation of the Council of Europe (EDQM) regarding metals (Metals and Alloys used in food contact materials) is available on the on Council of Europe website. The Nordic Council of Ministers has also prepared a user-friendly publication TemaNord 2015:552 on metals.
The USA (FDA) has adopted some regulations in this field and these may be used as a reference where EU-level legislation, EU Member States’ national legislation or guidelines from the authorities are unavailable. The FDA’s food contact material regulations can be found on the FDA website. Where EU-level legislation, EU Member States’ national legislation or guidelines from the authorities exist, FDA regulations alone may not be used as a safety reference.
4 Food contact material operator´s quality management system/ own-check control
Under Regulation (EC) No 2023/2006 (GMP Regulation, or Good Manufacturing Practice Regulation), companies shall have in place a quality management system which covers knowhow, quality assurance, quality control, and documentation in their own business sector. In practice this means the same as the own.check control obligation for operators in the food contact sector under the Finnish Food Act. Besides manufacturers, the Regulation applies to all stages of the supply chain of articles, including e.g. importers, marketers and wholesale distributors, or in other words, to all operations in the FCM sector.
The quality management system /own-check control does not require the approval of the authority and nor does it need to be based on quality management standards. Good manufacturing practice requires the operator and control authority to discuss the plan and the control authority may make comments and suggestions regarding the adequacy of the plan. Not all parts of own check control need to be in written form, especially in small-scale food contact material operations.
GMP Regulation specifies e.g. the following requirements for operators regarding good operating practice:
- Adequate knowledge of legislation pertaining to the FCM sector, of own process and other details relevant to risk management, as well as assurance and maintenance of personnel competence.
- A quality management system, including a quality assurance system (hazard analysis, process management, setting criteria for end product, and defining control points),
- Monitoring of implementation of quality assurance (monitoring process control points, corrective actions, inspection of final product)
- Recording of data and documentation, and submission of documents,
- Implementation of traceability.
Under the Finnish Food Act, operators in the food contact material sector are obliged to initiate the withdrawal of non-conforming products in compliance with Article 19 of EU's General Food Regulation (EC) No 178/2002[3], similarly to operators in the food sector. Operators must, in their own-check control, have the agreed procedures for the withdrawal of food contact materials.
Particular attention shall be paid to the special requirements laid down in the Annex to the GMP Regulation on the use of printing inks on the non-food contact side of a material or article (measures to be taken to avoid the so-called set-off phenomenon) and to the additional requirements laid down on the quality management system of a recycled plastic material process.
Printing inks intended for the non-food contact side of a material or article shall be formulated and/or applied as well as stored finished and semi-finished in such a way that substances from the printed surface are not transferred to the food-contact side through the substrate or directly or indirectly in the stack or reel at concentrations that exceed the quantity of the relevant substances permitted in food under Article 3 of Regulation (EC) No 1935/2004.
The printed surfaces may not come into direct contact with food.
The quality management system for plastic recycling processes shall guarantee adequate confidence in the ability to verify in the process the requirements set for the compliance of recycled plastic. The quality management system for plastic recycling processes shall include, in particular:
- a quality policy manual, containing a clear definition of the recycler’s quality objectives, the organisation of the business and in particular the organisational structures, the responsibilities of the managerial staff and their organisational authority where manufacture of the recycled plastic is concerned;
- the quality control plans, including those for input and recycled plastic characterisation, suppliers’ qualification, sorting processes, washing processes, deep cleansing processes, heating processes, or any other part of the process relevant for the quality of the recycled plastic including the choice of points which are critical for the quality control of the recycled plastics;
- the managing and operative procedures implemented to monitor and control the whole recycling process, including the inspection and quality assurance techniques at all the manufacturing stages, especially the establishment of critical limits at the points which are critical for the quality of the recycled plastics;
- the methods of monitoring the efficient operation of the quality system and in particular its ability to achieve the desired recycled plastic quality, including control of products which fail to conform.
5 Registration notification of food contact material operations to the food control authority
Since 2010, operators who place FCMs on the market have had an obligation to notify their operations and their place of business to the local food control authority. This is provided for in section 13 of the Finnish Food Act (297/2021). The registration obligation applies to all operators in the sector [manufacturers, importers (as well as third country and internal market imports) as well as wholesale distributors], excluding the retail stage. However, if a retail store has its own internal market or third country imports, they must submit an import registration notification. The notification obligation also applies to FCM importers or online stores engaged in wholesale sales of FCMs who do not have their own warehouse or sales premises. The control unit makes a charge in accordance with the tariff it has approved for processing the registration notification.
New companies or companies that have significantly changed their operations must submit a registration notification no later than four (4) weeks before the start of operations. Notification must also be given without delay of the suspension or discontinuation of operations. Notification is primarily made through the Environmental health care e-notification service (Ilppa) or by contacting the local municipal food control authority. The contact information of the local food control authority can be found on the Finnish Food Authority website (pick the municipality from the dropdown menu).
The authority must enter information about the operator and operations in the centralised national resource planning and information management system (VATI) of Environmental health care and notify the operator either electronically or in some other similar way that notification has been received and the information saved.
Authorities should actively identify operators in the sector in their own control area that have not yet submitted a notification and request them to do so. Instructions and forms for the submission and registration of notifications are available on the Finnish Food Authority website. It is recommended that the control authority post the instructions on submitting the notification in the e-notification service Ilppa and the form on their own website to make them available to operators.
Food establishments who import FCMs for their own use (i.e. to pack the food they manufacture themselves) no longer need to submit a separate notification of the import of FCMs. This activity is controlled separately in conjunction with food establishment control in accordance with 14.1 of the Oiva inspection guidelines.
Companies are informed about the obligation to submit a notification also on the "Enterprise Finland" website of the Ministry of Economic Affairs and Employment.
Importance of registering objects of control
The notification and registration obligation makes all types of operators in the FCM sector aware of their responsibility in terms of food safety, and equally known to the authorities. Official control requires the authorities to have adequate information about operators and the premises where operations take place. The measure also helps the authorities to target control. Besides control, registration also enables more efficient provision of information to operators.
Where an operator has operations in more than one location, a registration notification must be submitted to the authorities in each of the municipalities where operations take place. For example, where an FCM importer is headquartered in the area of one control unit and a logistics warehouse for operations in the area of another control unit, a registration notification must be submitted to both control units if documentation or quality management measures involving operations are carried out in both places.
Logistics warehouse activities related to imports or wholesale distribution may also be an outsourced service maintained by another operator and purchased by the actual FCM operator. An operator may centre the measures for the compliance of FCMs and the documentation traffic on the same place, i.e. the import logistics warehouse. In this case, the head office need not necessarily submit a separate registration notification. However, the control of FCMs cannot be carried out comprehensively as a head office control alone and, for example, ensuring traceability and the practical implementation of labelling requires control also in the logistics warehouse.
However, overlapping systematic control of the same matters at different sites of the same registered operations must be avoided. Where operations are located in the area of more than one control unit, the control units shall agree among themselves how systematic control is to be shared so that the operator does not incur extra costs arising from overlapping systematic control. This does not preclude control from being carried out at all operating sites where this is considered to be appropriate for the control of compliance.
Points 6-9 of these guidelines provide more detailed information on the control of FCM objects.
5.1 Exceptions to food contact materials operations
5.1.1 Food establishments that blow-mould pre-forms or deep-draw trays at the packing stage
Food operators who just mould a package from a plastic sheet or produce the final FCM from preforms just before the filling stage (e.g. deep-drawing of plastic trays or blow-moulding of PET bottles) are, as a rule, not considered to be FCM operators. On the other hand, suppliers of the materials (e.g. suppliers of preform bottles) must register as FCM operators. In addition, the material supplier shall provide precise instructions to the food business operator for the moulding of the material into the final FCM (e.g. data on moulding temperature and data) to ensure the compliance and safety of the final material. The food business operator shall comply with the instructions. A food operator deviating from the instructions is responsible for ensuring the suitability of the material and for having tests carried out on the materials regarding e.g. compliance.
5.1.2 3D printing in food establishments
Food business operators who produce e.g. parts of equipment or other FCM using 3D printing in compliance with the supplier of the printer and the raw material need not, as a rule, register as an FCM operator either. This requires the supplier of the printer to be a registered FCM operator and, besides supplying the printer, to also supply the raw materials and to have verified that the final product to be printed and used by the food business operator complies with requirements and is suitable for the intended application.
The printer supplier must provide precise instructions to the food business operator for the use of the device and the raw material (e.g. instructions on printing temperature and time, and quantity of raw material), and the food business operator must comply with the instructions provided. The instructions should also include a photo of the product the compliance of which has been verified by the printer supplier. The instructions shall, on request, be presented to the control authority.
Should the food business operator deviate from the instructions provided (e.g. uses the printer to produce other products which the printer supplier has not verified to be safe, or procures raw materials from elsewhere), this exemption from registration and verification of compliance will no longer apply. The food business operator is then also considered to be an FCM operator and must, among other things, verify the compliance of the printed article through testing.
6 Objectives in the control of food contact materials
The objectives of the requirements laid down for FCMs are above all intended to protect consumer health by ensuring that the FCMs on the market are safe and otherwise in compliance, prevent the misleading marketing of FCMs and to ensure the free movement of goods within the EU. Achievement of these objectives requires official control to be carried out as required by the Finnish Food Act (297/2021) and EU’s General Control Regulation.
Control is based on document control and on control of practical operations including process management. Due to the limited resources available, it is appropriate to target control using a risk-based approach.
6.1 Risk-based control in the food contact material sector
Practice has shown that all FCM operators are still not aware of the registration obligation and have not submitted their registration notification of their operations to the municipal food control authority as required under section 13 of the Finnish Food Act (297/2021). Authorities should actively work to get operators in their area added to the register of objects of control. First and foremost, registration is intended to make operators aware of their responsibility and obligation vis-à-vis the GMP quality management system in order to manufacture and place safe FCMs on the market. The authorities should also update the registration data on operators in conjunction with control visits.
The Finnish Food Authority recommends municipal control authorities to prioritise control of the FCM sector from the risk assessment perspective on the following points:
- control of achievement of compliance of FCMs manufactured in the authority’s own area and have a wide marketing base, and/or,
- are widely used,
- control of FCMs used in particularly demanding conditions (e.g. greasy products, hot conditions, long shelf-lives),
- operations manufacturing and/or using printing inks, lacquers, adhesives and recycled materials,
- new packaging innovations
- recycled materials and
- the registration of operations.
6.2 Risk-based control of food contact materials in the food sector
The Finnish Food Authority recommends that all control authorities (municipalities, veterinary inspectors, border veterinarians, the National Supervisory Authority for Welfare and Health (Valvira), the Finnish Defence Forces and Customs) prioritise control on the basis of risk:
- packaging of foods manufactured/packaged/imported and marketed widely in the authority’s own area,
- packaging materials used in particularly demanding conditions (e.g. greasy and hot foods, especially acidic foods) and
- new packaging innovations
- packaging which is further moulded in food establishments (bottles, deep-draw trays also 3D-printed FCMs)
- packaging containing recycled materials and
- components of manufacturing equipment (e.g. conveyor belts used with non-packaged food) containing polyvinyl chloride (PVC).
The food sector is primarily responsible for the suitability of materials, packaging, machinery, equipment and other articles intended to come into contact with food, as well as for ensuring that they are used in the conditions in accordance with the instructions issued. Control of use in the food sector also plays a significant role in opening the first link in the chain of compliance. Requests received by material suppliers from food businesses force FCM operators to actively provide answers to safety issues and to prepare declarations of compliance.
The Finnish Food Authority has prepared instructions for food business operators on how they should take into account the safety of FCMs in their own operations and in-house control. The instructions can be found on the Food Authority’s website.
6.3 Specialisation of the control authorities
It would be desirable for some control people in the control units to specialise in the inspection of operations in the FCM sector since control requires a knowledge of specific legislation where information must also be actively updated in line with changing legislation. The Finnish Food Authority hopes that control units encourage inspectors interested in this special subject to broaden their expertise competence. As a rule, control of the use of FCMs in food businesses in is based on procedures complying with the Oiva system, but even then specialisation in the control sector is useful.
The Finnish Food Authority plans, guides and develops control of the FCM sector. Each year the Authority arranges training for control officers and is involved in arranging training for operators. Participation in the control network of the Food Authority’s FCM control is a good way to deepen competence in FCM issues. From time to time, the Food Authority also arranges various control and research projects.
7 Priciples of official control in food contact material inspections
7.1 Municipal food control units
Municipal food control units control both FCM operations and the use of FCMs in food establishments in their respective area.
FCM sector: control of the achievement of the compliance of materials and articles at the places where they are manufactured and marketed:
- manufacturers of the actual materials and intermediates
- importers (imports from outside of the EU and from the internal market)
- wholesale distributors and marketing of FCMs.
Food sector: control of the correct use of materials and articles in places where food is manufactured or packed as well as in conjunction with the import of pre-packaged foods such as:
- places where food is manufactured, packing stations,
- mass caterers,
- points of sale of food,
- import of pre-packaged foods.
The approach in both of these sectors is that the authority inspects how the compliance of materials and articles in achieved in operations of the business and that they comply with the requirements of Regulation (EC) No 1935/2004.
An annual fee is charged for control as provided by the Finnish Food Act (297/2021) and a charge is made for each inspection in accordance with the tariff approved by the control unit. This charge may not be greater than the costs of the inspection.
Competent control authorities for inspections in the food sector:
- municipal control authorities; the use of materials and articles in places of primary production, manufacturing, food presentation and catering establishments controlled by the municipality,
- Finnish Food Authority’s veterinary inspectors; the use of materials and articles in slaughterhouses and associated facilities,
- border veterinarians; control of achievement of FCM requirements for pre-packaged food of animal origin imported from outside of the EU,
- environmental health authorities of the Finnish Defence Forces; the use of materials and articles in food sector establishments within the Defence Forces
- National Supervisory Authority for Welfare and Health (Valvira); control of the use of materials in places where alcoholic beverages are manufactured and handled as well as control of the compliance of packaging materials of alcoholic beverages of products imported from outside of the EU and from the internal market.
7.2 Customs
Customs controls the achievement of FCM compliance in conjunction with imports from outside of the EU and from the EU internal market. Customs are also tasked with the control of compliance with the specific import conditions required by Regulation (EU) No 284/2011 laying down specific conditions for the import of plastic kitchenware originating in or consigned from the People’s Republic of China and Hong Kong Special Administrative Region, China. The Regulation applies to polyamide (nylon) and melamine kitchenware intended to come into contact with food as the tableware has been found to release into food, on a recurring basis, levels of chemicals (primary aromatic amines and formaldehyde) that are harmful to health and which exceed the limits authorised by EU legislation. Typical articles intended by the Regulation include ladles, spoons, whisks, plates, cups, bowls and children’s tableware.
In addition, Customs controls the achievement of FCM requirements for pre-packaged food of non-animal origin or multi-ingredient foods imported from outside of the EU and from the internal market.
8 Inspection of food contact material operations
8.1 Risk classification of FCM operations and determination of control frequency
Guidelines for the risk classification and determination of the need for controls of packaging and other FCM operations are included in the Finnish Food Authority’s risk classification guide 10503 (“Food establishment risk classification and determination of the need for controls”).
The guide divides the points in the FCM sector into three groups depending on the scale of operation:
- small and medium-scale operations,
- large-scale operations
- very large-scale operations.
The scale of FCM operations is determined according to the following indicators:
Risk classification 1– small or medium-scale food contact material business
- Material qualities 1–3
- Production volume < 100 pcs/year < 10,000 kg/year
- Floor area of production and storage facilities < 100 m2
- Staff volume < 15
- Turnover < €2m/year.
Risk classification 2– large-scale food contact material business
- Material qualities 4–8
- Production volume 100–1,000 pcs/year 10,000–1m kg/year
- Floor area of production and storage facilities 100–500 m2
- Staff 15–100
- Turnover €2m–€10m/year.
Risk classification 3 – very large scale food contact material business
- Material qualities > 8
- Production volume > 1,000 pcs/year > 1m kg/year
- Floor area of production and storage facilities > 500 m2
- Staff volume > 100
- Turnover > €10m/year.
If a business fulfils three criteria of a certain classification, that classification is chosen for it. If three criteria are fulfilled in none of the classifications, the appropriate classification is chosen based on the amount of material qualities.
If the ranges include food contact materials intended for small children (e.g. baby bottles, baby food packaging, etc.) the risk classification is always 2 or 3.
Also, if operations include manufacturing, the risk classification is, as a rule, elevated by one, for example, from risk classification 1 to risk classification 2 and from risk classification 2 to risk classification 3.
Relief from the fee for control and regular inspection for small-scale and low-risk FCM operations
Small-scale and low-risk FCM operations are exempt from the control service charge and from regular inspections of operations. NB! They are still subject to registration notification to the municipal food control authority, please refer to the guidance in section 5. Moreover, the municipal food authority must provide them with advice and guidance as necessary.
Where the operations of a business come under risk classification 1 in respect of all criteria and the annual turnover of food contact materials is below the turnover threshold for VAT purposes, the concept of small-scale FCM operations applies to low-risk FCM operations, such as to the following operations:
- artisanal businesses such as small pottery workshops, metal workshops and workshops making articles from wood,
- cutting FCM into sheets, i.e. small-scale processing of FCM which does not involve printing,
- small-scale internal market imports and wholesale.
Third-country imports or operations which involve printing with printing inks cannot be considered as being low risk, which means that the relief from the fee for control does not apply to such operations. More information on the fee for control can be found in the Finnish Food Authority guide (add link).
Examples of risk classification, inspection frequency and determination of the fee for control:
Example 1: The establishment has 2 material qualities, their production volume is 10m kg/year, floor area of production and storage facilities is 70 m2, staff volume is 5 and turnover is €5 million/year; the appropriate risk classification is 1. However, because the business includes manufacturing, the classification is elevated by one, so the final risk classification is 2.
Example 2 The establishment has 5 material qualities, their production volume is 1,500 pcs/year, staff volume is 14, floor area of production and storage facilities is 200 m2 and turnover is < €2m/year; none of the risk classifications is chosen because three criteria are not fulfilled. Therefore, risk classification 2 is chosen based on the number of material qualities.
Example 3: Small-scale metal workshop or pottery workshop. All the information describing the nature and scope of operations used in classification of the site complies with risk classification 1. In addition, the annual turnover of contact materials is below the turnover threshold for VAT purposes, even though manufacturing is involved. No fee for control is charged and no regular inspections are made. Inspections are made as necessary.
Example 4. Small-scale printing and stamping of cardboard boxes. All the information describing the nature and scope of operations used in classification of the premises complies with risk classification 1. In addition, the annual turnover of contact materials is below the turnover threshold for VAT purposes. The risk classification 1 is chosen. A fee for control is charged because operations include printing with printing inks. Regular inspection is carried out at least every three years.
The operator must provide information about the scale of operations as part of the basic information in the notification of operations and the information is checked in conjunction with inspections to ensure they are up to date. The inspection frequency in risk classifications varies: in risk classification 1 it is 0.35 times a year, in risk classification 2 it is 0.5 times a year and in risk classification 3 it is once a year. The inspection frequency in risk classifications varies and may be 0.35, 0.5 times or once a year.
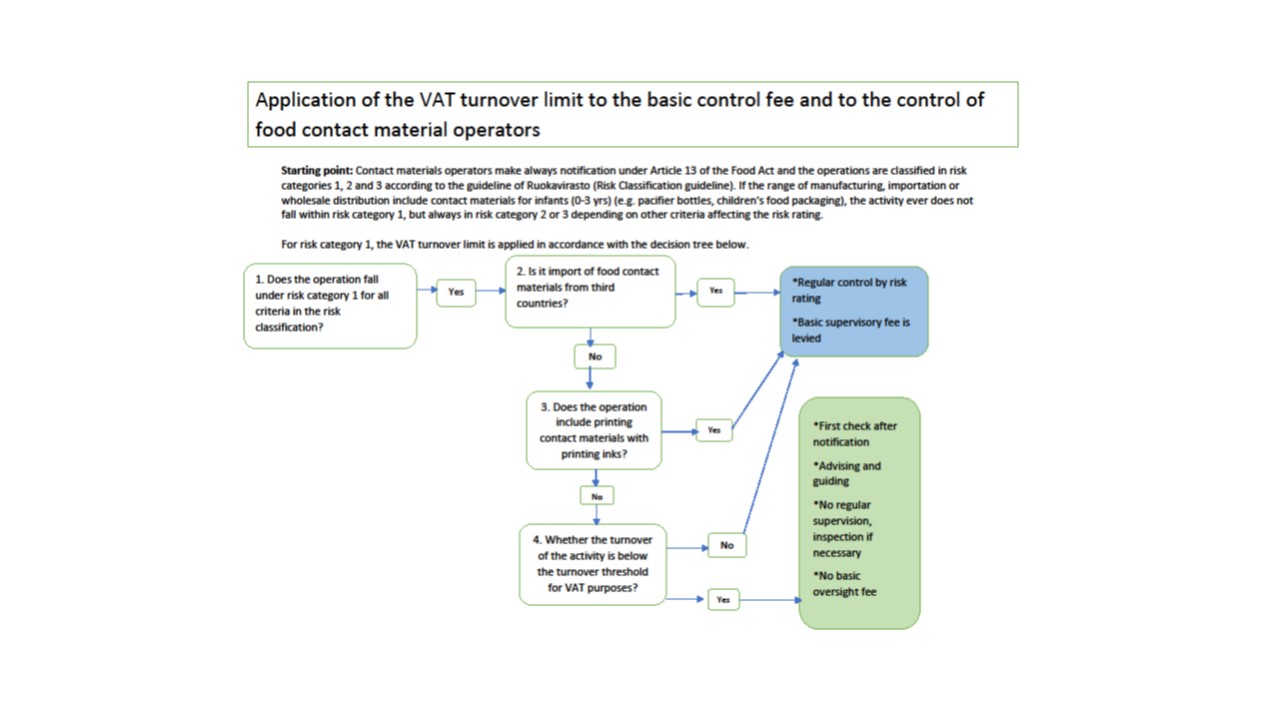
Figure 1. Decicion tree of how the application of the VAT turnover limit to the basic control fee and control levied on contact material operators.
8.2 Authorities and duties of the control authorities
The competent control authorities in inspections in the FCM sector are the municipal control authorities and the Customs ((importers).
In Finland, control of the achievement of FCM compliance complies with the Food Act (297/2021). The Food Act grants municipal food control authorities the same rights, duties and authority in the places of business in the FCM sector as they have in food businesses, the right to:
- access the premises,
- carry out inspections,
- access information and written documentation, including confidential information, for control purposes
- take samples and
- initiate administrative measures, where necessary.
The duties of the Customs are provided for separately and more information about these can be found on the Customs website.
Likewise, the provisions of the Food Act apply concerning any administrative coercive measures. The Finnish Food Authority has published "Guide on the use of administrative coercive measures in food control pursuant to the Food Act” (In Finnish and Swedish only).
Also Article 24 of the Framework Regulation and Article 5 of the EU Regulation on official controls (2017/625, Control Regulation) require the authorities to regularly inspect materials and articles intended to come into contact with food. The places of manufacture, the premises of the importer, the premises of the distributor and those food establishments where the materials and articles are used in contact with food are the most appropriate locations to carry out these inspections. The retail sale of FCMs is not regularly controlled but where necessary control may be targeted in conjunction with consumer complaints, for example.
8.2.1 Right of the control authority to access documents
The Declaration of Compliance/Statement of Confirmation) is a public document between the operators and as such must naturally also be accessible to the authority. Article 16 of the Framework Regulation requires the operator to make available appropriate (supporting) documents to demonstrate compliance. Such documents include e.g. composition data on the material, toxicological information on the substances and the results of migration tests. These ‘background’ documents must be available to the competent authorities on request but in operations carried out between companies they are usually classified as business secrets. Article 7 of the GMP Regulation (EC) No 2023/2006 on good manufacturing practice for FCM also requires the manufacturer to make all documents available to the competent authorities at their request.
Risk analyses carried out to verify the achievement of compliance and laboratory analyses are the operator’s responsibility as part of the GMP system in-house control. Some of the migration tests conducted in a laboratory can be replaced by mathematical calculations. The results of risk assessment, the test results from samples and any mathematical calculations must, on request, be made available to the authority.
8.3 Inspection by a municipal food control authority in a place of FCM manufacture
The inspection reviews both the implementation of the operator’s quality management (in-house control) in practice and the implementation of the compliance of the products. The inspection requires familiarisation with the legislation and Finnish Food Authority’s guidelines applying to the sector. The FCM-BTSF (Better Training for Safer Food) courses provided by the EU Commission also provide good training for control in the FCM sector.
Inspections primarily focus on the practical implementation of the operator’s quality management, which refers to the inspection of the implementation of the chemical compliance of the products. Before setting out to do the first inspection, the control authority should ask the operator to submit the GMP quality management system/in-house control system plan for a pre-review. It is also useful to study the company’s website in advance in order to obtain an overall picture of the company’s operations.
The Food Authority has prepared an inspection form for the inspection of the implementation of own control and the compliance of products in the FCM sector: Form 1.Inspection form for the inspection of manufacturing of food contact materials.
The control authority can use the form in the inspection to assess how the company’s quality management works and how quality assurance is implemented in practice. If unclear issues or shortcomings are found in quality assurances, there is cause to check how the procedures concerned are recorded in the GMP quality management programme.
The inspection form comprises the following seven topics (numbered subheadings), which are
- management of the composition of manufactured products
- tests carried out on the products manufactured
- declarations of compliance or other compliance documents prepared for products manufactured
- labelling on FCMs and articles marketed to consumers
- traceability
- processing methods/processes.
Below each topic a checklist is provided of points included in the assessment of that sub-area and which are answered YES, PARTLY or NO.
The topics are assessed on a four-grade scale A, B, C and D:
- = compliant
- = good, minor deficiencies might exist
- = corrective action required, deficiencies/shortcomings that jeopardise food safety and which must be corrected within a deadline
- = poor, deficiencies/shortcomings that jeopardise food safety and which must be corrected immediately.
Instructions for assessment have been prepared to promote fair assessment. The instructions are currently being piloted (until 30 March 2022). The control authorities can access the instructions in the FCM control unit’s workspace Pikantti (authorities’ extranet system maintained by the Finnish Food Authority). The instructions have also been forwarded to the industry for comment and can be viewed on the Food Authority website (in Finnish only).
The topics assessed carry different weightings from the food safety perspective. Assessment of the whole operation is carried out from the viewpoint of risk assessment taking into account the scope and nature of operations. For the purposes of control of the processing methods/processes section, the Finnish Food Authority has prepared an internal guide 17067, which the control authorities can also access in the FCM control unit’s workspace Pikantti. The guide has not been published in the Food Authority website.
There is reason to inspect all the points on the inspection form on the first inspection. In subsequent inspections, it is not always necessary to inspect all the topics on each inspection, but to focus on some of the matters covered by the inspection form and to check them more thoroughly, for example, some products or some process stages.
8.4 Inspection of FCM operations in municipal food control at other stages in the distribution chain (import, marketing, wholesale, incl. “virtual operations”)
Article 2 of Regulation (EU) 2023/2006 is applied to all sectors and to all stages of manufacture, processing and distribution of FCM, up to but excluding the production of starting substances. This means that, for example, import companies and operators engaged in internal market trade are required to have the applicable parts of a GMP quality management system/in-house control plan in place.
In practice, this mainly means that the importer/marketer/wholesale distributor:
- knows on a general level the requirements specified for FCM in their sector,
- verifies from suppliers the compliance of articles intended to come into contact with food and obtains documents to demonstrate this,
- attaches the documents demonstrating compliance with onward deliveries,
- ensures the correctness of labelling and
- manages FCM traceability.
The Finnish Food Authority has prepared an inspection form for inspections at importers and wholesale distributors Liite 2.Inspection form for the inspection of the import and distribution of food contact materials
Assessment is made using the same four-grade scale A, B, C and D as in assessment of FCM manufacturer and the assessment results for the control object are stored in the VATI system, the joint control data system of Environmental health care.
8.4 Recording municipal food inspection control results in VATI
The control results for each sub-area are recorded in the VATI system, the joint control data system of Environmental health care. User instructions for VATI can be found on Pikantti, the extranet maintained by the Finnish Food Authority.
In its report of the inspection object, the control authority states the result of the inspection and any points requiring actions, requests the operator to make corrections and issues a deadline to make any corrections.
Operators who operate in both the FCM sector and food sector (e.g. a wholesaler or importer) must ensure that the results of operations in the FCM sector do not affect the results of the food sector operator’s Oiva inspections (see Section 8 for more details).
9 Inspecition of food contact materials in food sector establishments
Foods are packed in or otherwise come into contact with materials in all types of food sector establishments from primary production to food establishments such as slaughterhouses, food manufacturing plants, points of sale as well as catering establishments and kiosks. When inspecting the effectiveness of in-house control of a food industry operator, the control authority must also pay attention to the achievement in practice of compliance of the combination of food contact material/food product (packaging materials, production plant, machinery, equipment, utensils).
The basic criteria applied to packaging materials and other FCM are based on the properties of the food (e.g. fat content, moisture, pH value, alcohol content), the manufacturing process (e.g. contact temperatures in the production plant and when used by the consumer) and the shelf life.
Municipal control authorities and the Finnish Food Authority’s veterinary inspectors carry out inspections in accordance with the instructions in point 14.1 in the Oiva system. Oiva inspection instructions for both registered and authorised food establishments can be found on the Oivahymy.fi website. Inspection results are stored in the VATI system of Environmental health care and a total assessment of the establishment is published in the Oiva report.
The control of wholesale operators must take into account the fact that if the wholesaler operates in both the FCM sector and the food sector, the control of operations in the FCM sector will not be included in Oiva control and that any deficiencies found in this inspection may not affect the wholesaler’s Oiva grade. The Oiva control of a wholesaler takes into account the FCMs used in the wholesaler’s own operations not the materials resold to food business operators. Inspection of FCM operations focuses on control of the compliance of FCMs which the wholesaler distributes to its own customers.
Control authorities other than municipal and the Food Authority’s control authorities can use the Oiva system inspection guideline to help in their inspections. However, the results of these inspections are not published in the Oiva system but are stored in their own information systems.
Entry into force
- This guide has entered into force on 15 June 2021 and it replaces the earlier version (Finnish Food Authority 17018/6, published on 1 August 2020).
Updates in version 7
- Updated the number of the new Recycled Plastics Regulation to paragraph 3.1 of the Guide;
- Updated web links to section 3.2 of the guide (deleted and added a link to Food Authority website where they can be found).
[1] Regulation (EC) No 1935/2004 of the European Parliament and of the Council on materials and articles intended to come into contact with food and repealing Directives 80/509/EEC and 89/109/EEC
[2] Commission Regulation (EC) No 2023/2006 on good manufacturing practice for materials and articles intended to come into contact with food
[3] Regulation (EC) No 178/2002 of the European Parliament and of the Council laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety,